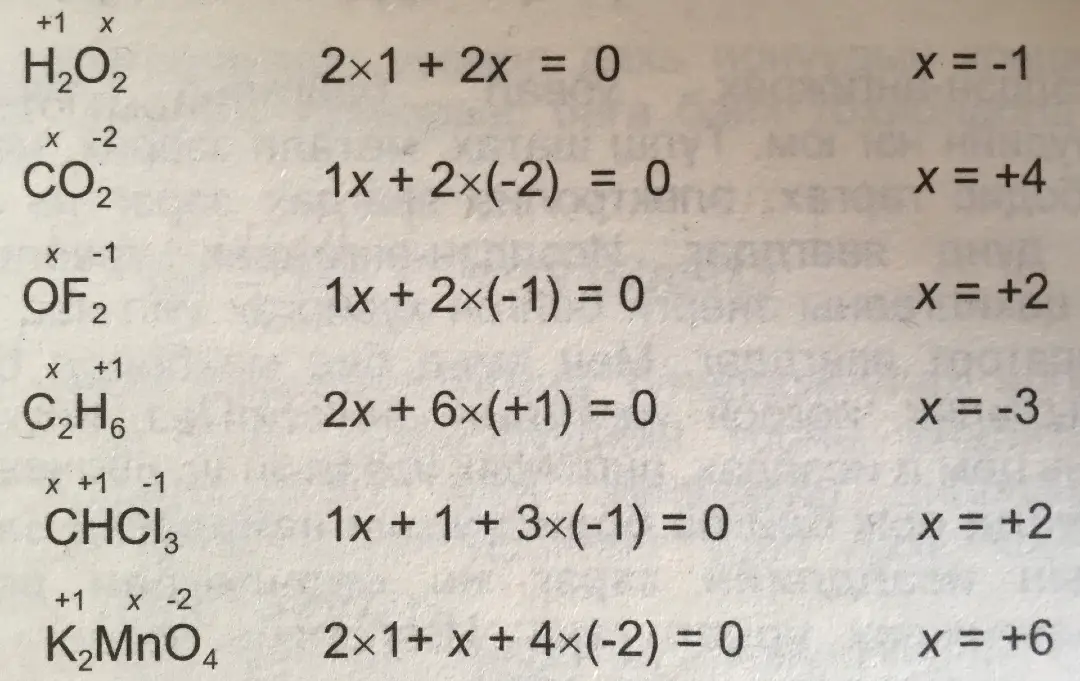Hello, guys, In today’s this article we will study about Oxidation And Reduction. Like – what is Oxidation And Reduction? What are it, rules and concept We will know the answers to many such questions today, so let’s start.
What are Oxidation and Reduction?
Oxidation and reduction are two major events that occur in any chemical reaction. Which are explained according to the following concept.
Old Concept –
According to the ancient concept, when hydrogen-positive element metals are added in a chemical reaction or oxygen-negative electronegative elements and non-metals are released, then such a process is called reduction.
Example –
C + 2H2 ————- CH4 (reduction)
In a chemical reaction, when an oxygen atom, negative electronegative element, and non-metal are added or a hydrogen atom, positive element, and metal are released, then such a chemical reaction is called oxidation.
Example –
C + O2 ———– CO2 (oxidation)
“The ancient concept fails later on, but the study of oxidation and reduction in organic chemistry is done from the ancient concept”.
Example –
alcohol, aldehyde or ketone acid, or ester
R-CH2-OH ———- R-CHO ———- R-COOH (oxidation)
2- Electronic Concept –
According to electronic theory, the process of taking or giving up one or more electrons by a molecule, atom, or ion is called oxidation.
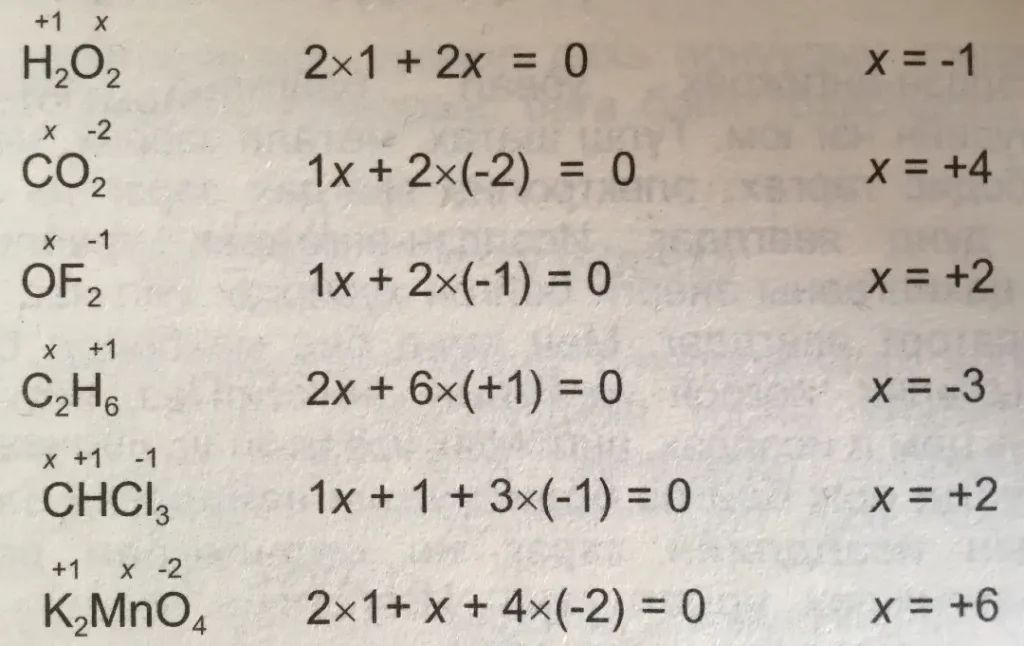
The valency of an element increases in the process of oxidation. This increase in valency is equal to the number of electrons discarded.
Na ————- Na+ + 1e-
Cu ————- Cu+2 + 2e-
Zn ———— Zn+2 + 2e-
Fe ————- Fe+3 + 3e-
In the oxidation process, because electrons are lost, this process is called the loss of electrons or De-Electronation.
According to electronic theory, the process of accepting one or more electrons from a molecule or atom is called reduction. In the reduction process, the valency of an element decreases. This decrease in valency is equal to the number of electrons gained.
Cl2 + 2e- ——— 2Cl–
Sn+4 + 2e- ——– Sn+2
Fe+3 + 1e- ——— Fe+2
In the reduction process, because electrons are taken away, this process is called Electronation.
Oxidation Number Method –
According to the concept of oxidation number, when the oxidation number of an element increases, then this process is called oxidation.
H2S-2 + oxidizer ——– S0
Sn+2Cl2 ———– Sn+4Cl4
According to the oxidation number concept, the reduction is the process in which the oxidation number of an element decreases.
(Potassium Permagnet) KMn+7O4 + H+ ——- Mn+2
(Potassium Chlorate) KClO3 ——– KCl–
What is the Oxidation Number?
The oxidation number of an element in a given compound is equal to the number of electrons gained or discarded in that element’s transition from the free state to the compound state.
Difference between oxidation number and valency
| Oxidation Number | Valency |
| The amount of charge that lies on an atom of an element is called the oxidation number. | The number of bonds formed by an atom of an element with another atom is called the valency of that element. |
| The oxidation number of an element can be positive, negative or zero, such as – Nitrogen has an oxidation number of -3 and NaCl has an oxidation number of +1. | The valency of an element is neither positive nor negative. For example, in PH3 the valency of P is 3 and in CH4 the valency of C is 4. |
| The oxidation number of the same element is different in different compounds. For example, inCH4, CH3Cl, CH2Cl2, CHCl3, and CCl4 the oxidation number of C is -4, -2, 0, +2, and +4 respectively. | The valency of an element is the same in different compounds. For example, in CH4, CH3Cl, CH2Cl2, CHCl3, and CCl4 the valency of C is 4. |
The rule’s for oxidation numbers –
The following rule is presented to find the oxidation number of an element.
Rule 1 – The oxidation number of the neutral compound is zero. Ex – CO, CO2, NO (Nitrosyl) NH3, H2O, C2H2, C2H4
C5H5N = Pyridine = Py
Rule 2 – When two electronegativity elements are joined together, then the element which has an electronegative oxidation number and the element which has less electronegativity. It has a positive oxidation number.
F>Cl>Br>I
Order of Electronegativity I+1 -Cl-1
Rule 3 – The oxidation number of an element in any compound is equal to the charge present in it, the sum of the oxidation numbers of all the elements present in the compound.
SO4-2 = sulfate
Let the oxidation number of sulfur (S) be x and the oxidation number is Y, then –
x + 4*y = -2
Rule 4 – All elements of the I-A group have an oxidation number of +1.
I-A Group = Li, Na, K, Rb, Cs, Fr
All the elements of the II-A group have an oxidation number of +2.
II-A Group = Be, Mg, Ca, Sr, Ba, Ra
All the elements in the III-A group have an oxidation number of +3.
III-A Group = B, Al, Ga, In, Tl = = +3
F fluorine element has maximum electronegativity and its oxidation number is always -L.
Rule 5 – The oxidation number of hydrogen in the normal state is +1.
The oxidation number of hydrogen in hydrides is -1. like –
In I-A Hydride, II-A Hydride, III-A Hydride, lanthanide, and actinide, the oxidation number of hydrogen is -1.
Molecular hydrogen The oxidation number of nascent hydrogen is zero.
H2 [H] = 0
Rule 6 –
Oxygen – (i) Oxidation number of oxygen is -2 in a normal state. This oxidation number occurs in oxides and oxic acids.
The oxidation number of oxygen in peroxides is -1. For example, the oxidation number of oxygen in H2O2, Na2O2 is -1.
Peroxide = O2-2
The oxidation number of oxygen in superoxide is -1/2.
Superoxide = O2-1
Example – KO2 Potassium Super Oxide
The oxidation number of oxygen in ozonide is -1/3.
Ozonide = O3-1
Ex – KO3 Potassium Ozonide
The oxidation number of oxygen in OF2 is +2.
OF2 = Oxygendifluoride
The oxidation number of oxygen in O2F2 is +1.
O2F2 = dioxygen difluoride
Molecular oxygen and nascent oxygen have zero oxidation numbers.
O2, O3 [O] = O
Rule 7 – The maximum oxidation number of an element is equal to the square number, while the minimum oxidation number is equal to the square number (-8).
Rule 8 – Average oxidation number of an element can be fractional, but the oxidation number of an element cannot be fractional.
N3H = Hydrozoic Acid
3N = 0 + 0 – 1
N = -1/3
N3H
3NH = 0
3N = – 1
N = -1/3
Rule 9 – The oxidation number of every element is zero in the free state. For example, the oxidation number of sodium and carbon is zero.
Rule 10 – In a compound in which the same atom is present, then the oxidation number of all the atoms is zero. For example – the oxidation number of S in S8, the oxidation number of P in P4, the oxidation number of H in H2, and the oxidation number of O in O3 is zero.
Rule 11 – The sum of the oxidation numbers of the elements present in every neutral compound is zero.
The oxidation number of the compound = Sum of the oxidation number of atoms of all the elements in the compound = 0
Rule 12 – Oxidation numbers of all metals in compounds are always positive.
Rule 13 – The oxidation number of neutral ligands in a hybrid compound is zero. For example – [Cu(NH3)4]SO4 has zero oxidation number of NH3.
Difference between oxidation and reduction
| Oxidation | Reduction |
| Hydrogen is released in it. | Hydrogen is added to it. |
| Oxygen is added to it. | Oxygen is separated in it. |
| In this, the ratio of the electric negative element increases. | In this, the ratio of the electric negative component decreases. |
| In this, the ratio of the electropositive component decreases. | In this, the ratio of the electropositive element increases. |
| In this, the valency of the electropositive element increases. | In this, the valency of the electropositive element decreases. |
| It increases the oxidation number. | It decreases the oxidation number. |
| In this, the electrons are separated. | It receives electrons. |
What is an oxidizing agent?
Oxidizing Agent –
Substances that gain electrons in a reaction or reduce themselves by oxidizing other substances are called oxidizing agents. In the process of oxidation, the oxidizing substance is reduced.
What is a reducing agent?
Reducing Agent –
Substances that give up electrons in a reaction or are themselves oxidized by reducing other substances, or that lose electrons or have an increased oxidation number. It is called a Reducing Agent. In the process of reduction, only the reducing substances get oxidized.
Friends, I hope you liked the given information about Oxidation And Reduction Friends, if you have liked this given information, then please share it with as many friends as possible so that they can also benefit from it.
Thank you