Hello friends, welcome to another new article in which we will talk about “What is Faraday’s law?” If you know about this rule well, then let’s start without wasting time.
What is Faraday’s law?
Faraday’s Laws of Electrolysis –
Michael Faraday did many experiments on electrolysis and presented the results obtained by two rules. These laws are called Faraday’s law of electrolysis. Following are these rules.
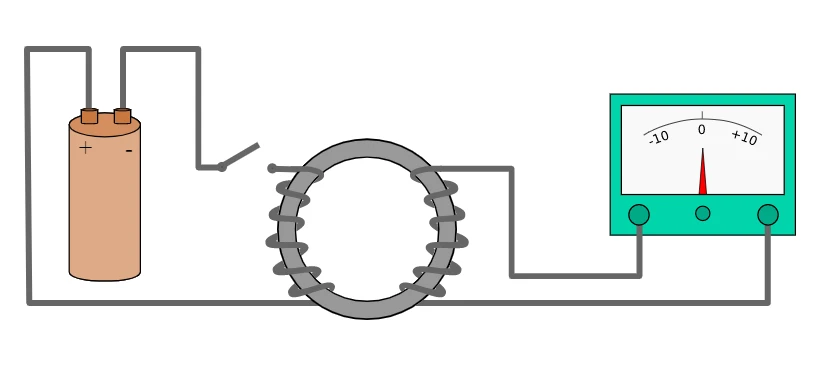
Faraday’s first law of electrolysis –
The amount of a substance deposited or released at any electrode is directly proportional to the amount of electricity flowing in the electrolytic solution.
Therefore, if ‘m’ grams of matter are deposited when Q coulombs of electricity are passed. Then
m Q or m = ZQ
Where ‘Z’ is the proportional constant and is called the electrochemical equivalent. If I ampere current is passed for t seconds, then –
Q = I × t
Thus, m = Z × Q = Z × I × t
Now if Q = 1C or I = 1 ampere and t = 1 second, then
With m = Z multiplied by 1 ampere and one second
or m = Z
Therefore, the deposited mass of a substance, when one-ampere current is passed for one second, that is, the amount of one coulomb of electricity is passed, is called the electrochemical equivalent of the substance.
(2) Electricity – Faraday’s second law of electrolysis –
According to this, when the same amount of electricity is passed through different electrolytic solutions combined in series. Then the weights of the substances produced on the electrodes are directly proportional to their chemical equivalent weights.
Example – When the same electric current is present in two electro-degradable solutions; For example, copper sulfate (CuSO4) and silver nitrate (AgNO3) connected in series are passed through. Then the weights of the deposited copper and silver will be as follows –
Weight of deposited copper / Weight of deposited silver = Equivalent weight of copper / Equivalent weight of silver.
However, in modern terms, the term equivalent weight is not used. Faraday’s electrolytic law can also be written in terms of moles of electrons regulated during an electrochemical change.
“Moles of the substance deposited or released, that is, the amount of chemical change that occurs, is proportional to the number of moles of electrons regulated during oxidation-reduction reactions.”
Thus, knowing the amount of electricity to be passed, we can find the number of moles of the product formed by a proper electrode reaction. Knowing the number of moles of the products, we can find their mass or volume in the gaseous state.
The conductivity of the solution of an electrolyte –
It is the inverse of the resistance R. and can be simply defined as the current through which current flows through a conductor.
c = 1/R = A / p(rho) l (since R = pl/A)
k = 1/p = 1/R × l/A
Here k is the specific conductivity.
The SI unit of conductivity is Siemens. Which is denoted by ‘S’ and is equal to ohm-1.
Molar conductivity –
The conductivity is shown by all ions when 1 mole of electrolyte is dissolved in a solution. is called the molar conductivity, it is expressed by lambda m. If V cm3 of electrolytic solution contains 1 mole of electrolyte, then.
Lambda m = k × V
= k × 1000 / molarity
= k × 1000 / M
Its unit is ohm-1 cm2 mol-1 or siemens cm2 mol-1.
Friends, this post “What is Faraday’s law?” Share this with your friends so that they can also benefit from it.
Thank you