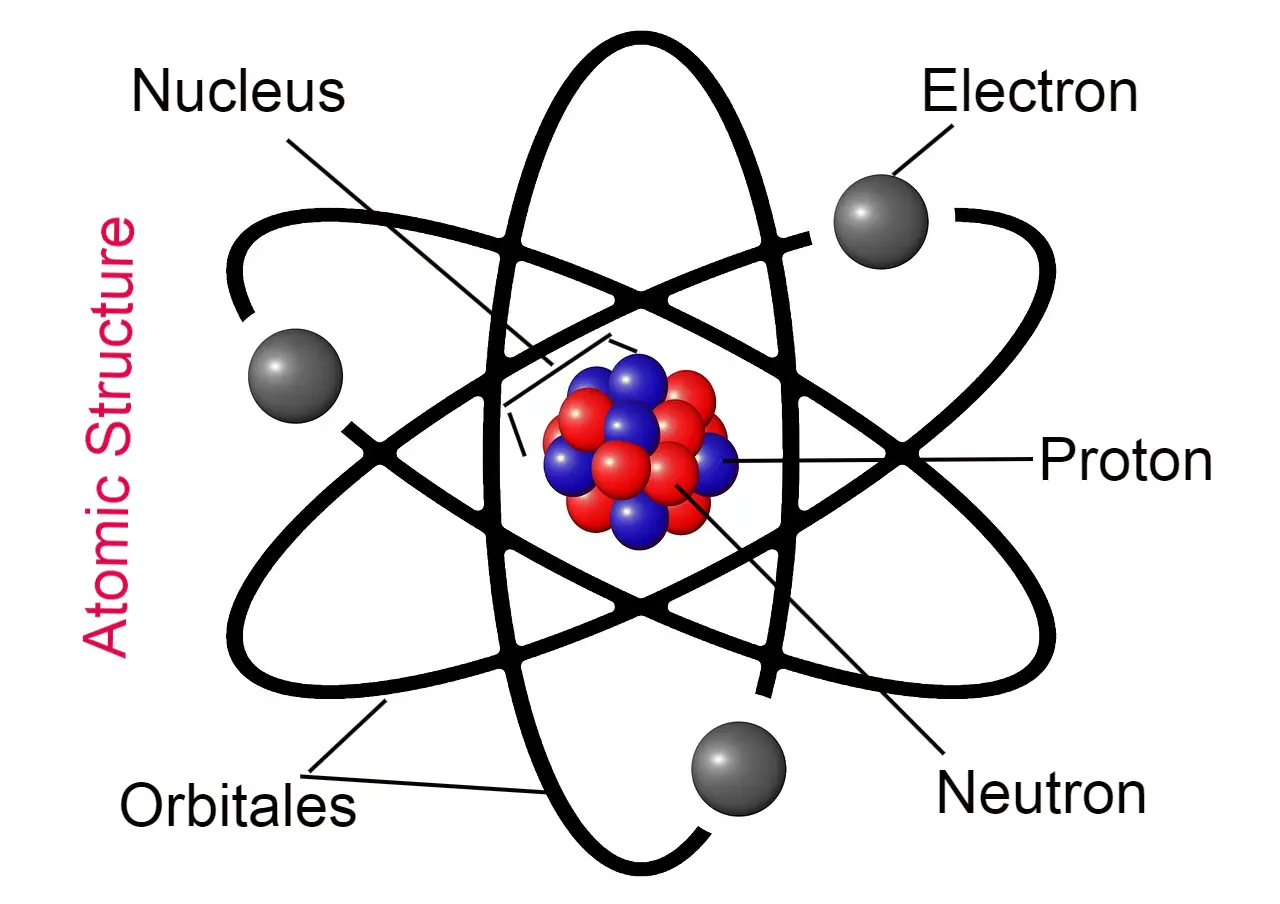
Hello friends, today we will read about the chapter atomic structure. Friends, in this we will learn about the atomic model. I have already written an article about atoms, so if you want to know about atoms, then you can read that post by clicking on the link.
What is atomic structure?
The structure of the atom is called the atomic structure, studying this structure only comes under the atomic structure.
Rutherford’s experiment –
Alpha Rays = Alpha Particles = 2He4 = Helium Nucleus
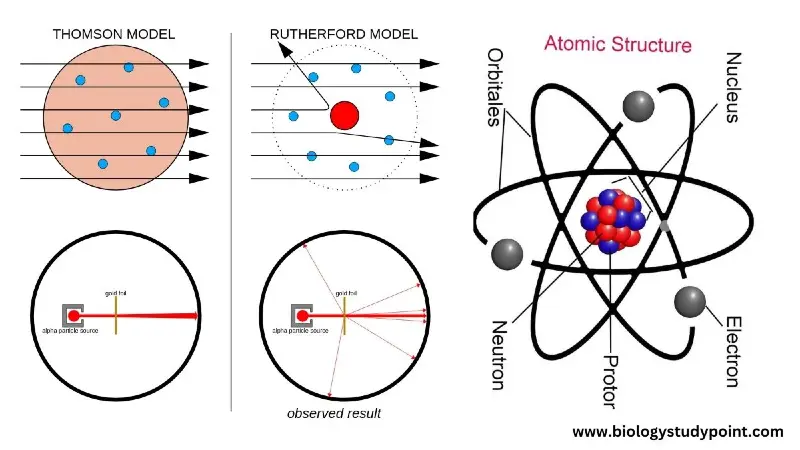
A scientist named Rutherford bombarded alpha particles on a thin metal foil (gold, silver), from which he made the following conclusions.
- Most of the alpha particle escapes straight away. From this, it was concluded that most of the atoms are hollow.
- When alpha particles are bombarded near the atomic center, the alpha particles deviate from their path. He clarified that there is a positively charged part in the center of the atom.
- When the alpha particle is bombarded exactly at the midpoint, one or two particles return to their path.
- All the mass of the atom and all the positive charges are concentrated or contained in the center of the atom. Which is called the nucleus of the atom.
- The total concentrated positive charge on the nucleus is equal to the electrons’ total negative charge so that the atom’s electric charge remains balanced and neutral.
- He said that the negatively charged electrons present in the atom keep revolving around its positively charged nucleus.
- The radius of the nucleus is 10-12 cm and the radius of the atom is 10-8 cm. From this, it is clear that the radius of the atom is about 10,000 times greater than the radius of the nucleus.
- This model of Rutherford was called the nuclear model of the atom. This model is also called the solar or planetary model because in this model it has been imagined that the way the planets revolve around the sun, in the same way, electrons keep moving around the nucleus.
- The Rutherford scientist thus discovered the atomic nucleus.
Classical Atomic Model –
The ancient atomic model is based on the particle property of the electron, in which the following model is presented.
Thomson Atomic Model –
The Thomson model is the first atomic model.
According to Thomson –
- The size of the atom is similar to that of a watermelon. In which the eating part is the nucleus and the electrons remain trapped in the form of seeds.
- The repulsive force between like charges and the force of attraction between opposite charges keeps the atom in a balanced state.
- He did not give any specific information about the atom, so later on this model failed.
Also, read – What is Chemistry? Complete information.
Rutherford Nuclear Model –
The Rutherford model is called the first nuclear model.
Atomic Structure Fa = Force of Attraction
Fc = Centrifugal force
All the mass of the atom and all the positive charges are concentrated in the center of the atom. Which is called the atomic nucleus. The electrons revolve in circular orbits around this nucleus. The force of attraction of the nucleus on the electron remains balanced with the centrifugal force acting on the outside of the electron.
According to Clark Maxwell, a scientist named Neil Bohr said that any accelerating charged particle continuously emits electromagnetic radiation. That should reduce the energy of the electron. As a result, the electron reduces its radius and while reducing it eventually falls into the atomic nucleus. But this does not happen. Thus, Rutherford’s atomic model fails.
Bohr’s Model –
Bohr’s model accepts the nucleus of Rutherford’s atomic model and makes modifications to Rutherford’s orbitals. According to this model, there are infinite energy levels around the atomic nucleus. Due to this, the electron orbits in a circular orbit towards the atomic nucleus, either accepting or giving up energy.
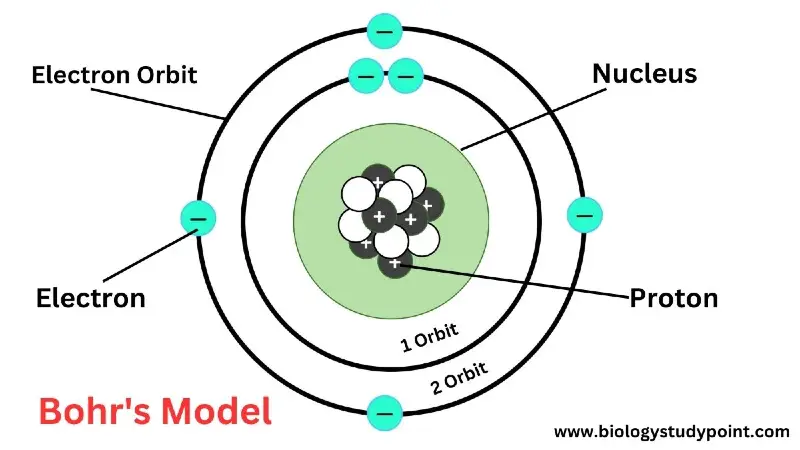
The electrons revolve in that orbit. Whose angular momentum is an absolute multiple of nh/2π?
mvr = nh/2π
where m = mass of the electron
v = velocity of the electron
r = distance of the electron from the nucleus i.e. radius
n = 1, 2, 3…integer number
h = Planck’s constant
The energy level in which the electron revolves. It is called the principal quantum number of the shell or orbital or principal energy level or stable orbital.
Electrons can change their orbits by absorbing or emitting energy.
ΔE = E2 – E1
n/ λ = E2 – E1
ΔE = change in energy
E2 = energy of higher energy level
E1 = energy of the lowest energy level
The principal quantum number and spectrum originated in the Bohr model.
Bohr’s atomic model applies to atoms or ions with a single electron.
Example – H, He+, Li+2, Be+3
Bohr’s atomic model does not explain the atomic structure of the atom, the Zeemann effect, and Stark effect, etc.
Radius, Velocity, and Energy –
According to Bohr’s atomic model, the radius, velocity, and energy of any electron in the nV orbital can be determined.
rn = n radius in orbit
rn = 0.529n2/ Z (in angstrom)
n = number of cells
z = atomic number of the element
The value of rn is in Angstrom.
vn = velocity of the electron in ‘n’ orbit
Vn = 2.188108z / n ohm / second En = energy of electron (e)- in nth orbital En = -RhcZ2 / n2
R = Rydberg Constant = Rydberg constant
R = 1.097107 m-1 (per meter) h = Planck’s constant h = 6.6710-34 joules – second
c = Velocity of light = 3108 m/sec Rhc = 2.1810-18 J/Atom
Rhc = 2.18*10-11 erg/Atom
Rhc = 13.6 eV/Atom
Sommerfeld model –
The Sommer field model was modified in Bohr’s orbits. According to this model, when the electron is near the nucleus, the orbit of the electron is elliptical and when the electron is away from the nucleus, its orbit is circular.
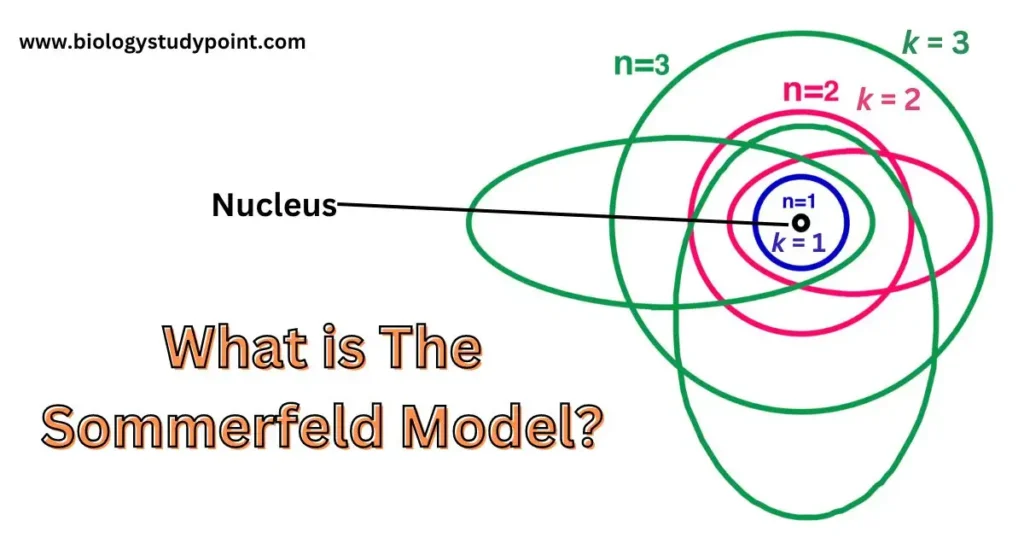
The angular momentum of an electron in a circular orbit is nh/2π. Whereas in an elliptical orbit, the angular momentum of the electron is kh/2π.
n = Principal Quantum number (shell) {Principal Quantum number (shell)}
K = Azimuthal Quantum number (subshell) {Bi-dimensional quantum number (subshell)}
Later on, the binomial quantum number K was changed to l.
The Sommer field model originated the binomial quantum number, which expressed the subshells of electrons.
Sommer field model explains the microscopic structure of atoms, the Zeemann effect, the Stark effect, etc. The disintegration of spectral lines in a magnetic field is called the Zeeman effect. Whereas, the disintegration of spectral lines in the electric field is called the Stark effect.
The ancient atomic model is based on the particle nature of the electron.
Modern Atomic Model –
The modern atomic model is based on the electron’s particle and wave nature. A scientist named de Broglie said an electron has particle and wave properties. Hence, it is clear that electron has a dual nature.
According to the mass-energy equation of Albert Einstein –
E = mc2 ———–(1)
According to a scientist named Planck –
E = hv
E = hc/λ (Elapsed v = c/λ) ————-(2)
V = New = Frequency of Light (Frequency of Light)
C = speed of light
Comparing with both (1) and other (2)
mc2 = hc/λ
mc2 = hc/λ
= h/mc
When a tiny particle is given the velocity of light, that particle produces waves.
V = speed of the particle
C = speed of light
C = V
= h/mv = h/p This is de Broglie’s equation.
= is the wavelength of light.
Thomson model, Rutherford model, Bohr model, Sommerfeld model, etc. The model is based on the particle of the electron. The angular momentum of an electron in the Bohr model is nh/2π.
Similarly, in the wave model of the electron, the angular momentum of the electron is nh/2π.
The length of a wave is called wavelength. If the electron produces n waves in one spin. So –
n wave length = nλ
The length of n waves is equal to the circumference of the circle.
n wave length = circumference of the circle
nλ = 2πr
where r is the radius of the circle.
= h/ mv
nh/ mv = 2πr
mvr = nh/ 2π
mvr = angular momentum of the electron.
Heisenberg’s Uncertainty Principal –
Momentum and position cannot be determined at the same time for any moving electron. It means either there is uncertainty in the momentum or there is uncertainty in the situation.
Δp = Δx h / 4π
According to Heisenberg’s equation, the product of uncertainty in linear momentum and uncertainty in position is either equal to h/4π or greater than h/4.
p = mv
Δp = m Δv
m delta v.Δx = h/ 4πm
According to this equation, the product of the uncertainty in the velocity of the moving electron and the uncertainty in the position is either equal to h/4πm or greater than h/4m.
Δp = uncertainty in the momentum of the moving electron
Δx = uncertainty in the position of the moving electron
Δv = uncertainty in the velocity of the moving electron
Conclusion –
Friends, let’s see. In this post we have learned about which things.
Well known about Rutherford’s Experiment, Ancient Atomic Model, Thomson Atomic Model, Rutherford Nuclear Model, Bohr’s Atomic Model, Radius, Velocity, Energy, Atomic Model of Sommerfeld, Modern Atomic Model, Heisenberg’s Uncertainty Principle.
So friends, if you liked the given information about atomic structure, then please share it as much as possible with your friends.
Thank you