Hello guy’s, In today’s article we are going to study Chlorobenzene. Like – What is Chlorobenzene? What are its physical and chemical properties, how is it made, what is its chemical name, and what is its structure? We will know the answers to many such questions today, so let’s start.
What is Chlorobenzene?
Chlorobenzene is an organic compound and it colorless good-smelling liquid heavier than water.
What is the Chlorobenzene Boiling Point?
The boiling point of chlorobenzene is 132 °C.
What Is The Chemical Name of Chlorobenzene?
The chemical name of chlorobenzene is chlorobenzene.
What Is The Molecular Formula Of Chlorobenzene?
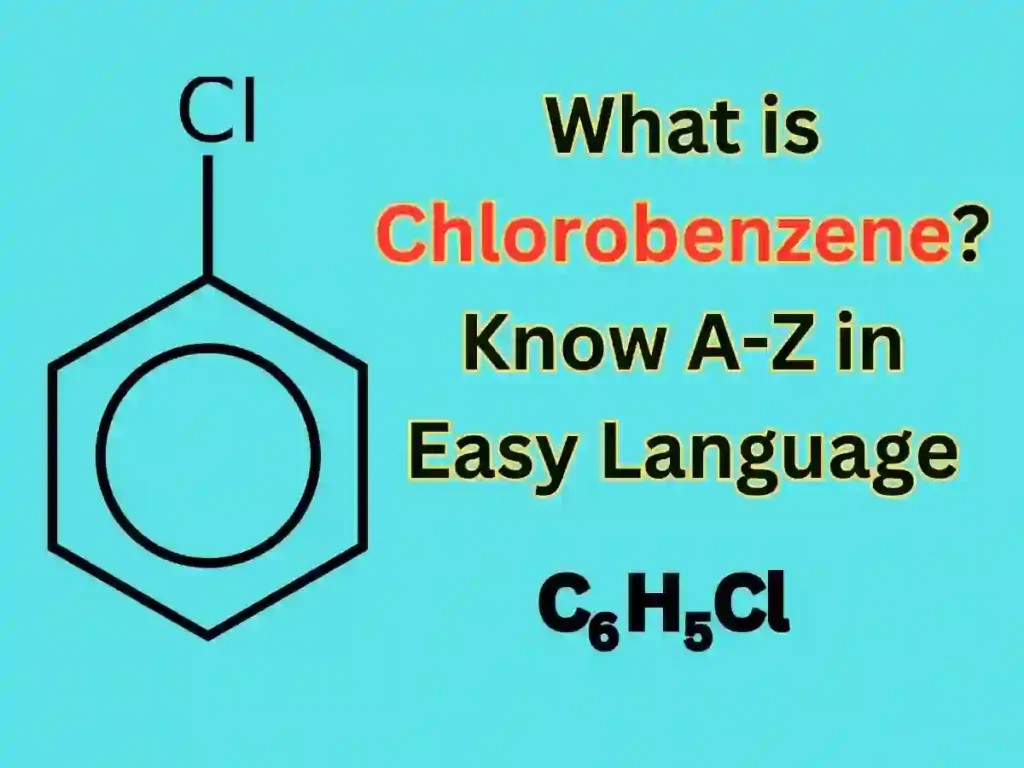
The molecular formula of chlorobenzene – C6H5Cl
What Is The Structure Of Chlorobenzene?
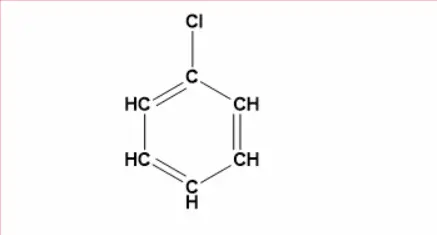
What Are The Methods Of Making Chlorobenzene?
The following is the method of making chlorobenzene.
Laboratory Method –
In the laboratory, chlorobenzene is prepared by heating benzene-diazonium chloride with cuprous chloride, and hydrochloric acid at 60 °C. This reaction is called the Schandmayer reaction.
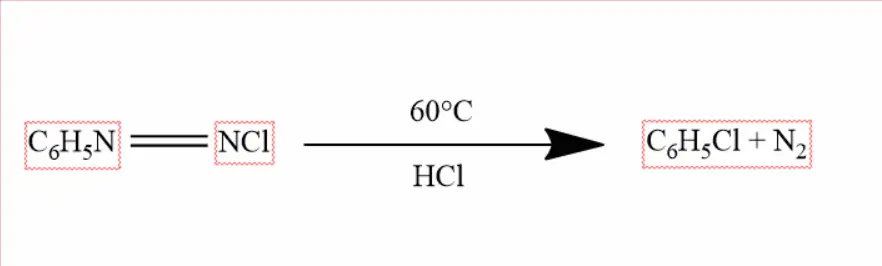
By Chlorination of Benzene –
Chlorobenzene is formed when benzene reacts with chlorine in the absence of light and in the presence of halogen elements iron or iodine.
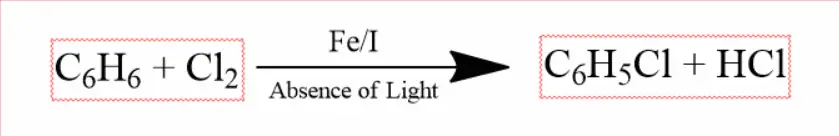
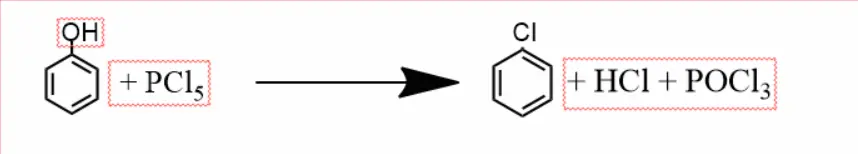
Phenol to Chlorobenzene –
Phenol reacts with phosphorus pentachloride to form chlorobenzene. And along with it, phosphoryl chloride is released.
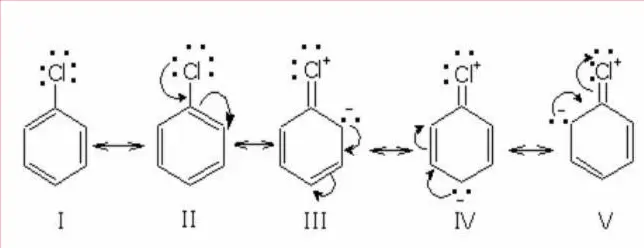
Resonance structure in chlorobenzene –
The Chlorobenzene molecule is a resonance hybrid of the following structures I to V.
Properties of Chlorobenzene –
Physical properties –
- Chlorobenzene is a colorless, sweet-smelling liquid. Its boiling point is 132 °C.
- Chlorobenzene is heavier than water.
Chemical Properties –
Reaction with Cuprous Cyanide (CuCN) –
Chlorobenzene reacts with cuprous cyanide and potassium cyanide in the presence of pyridine on heating at 200°C to form phenyl cyanide.
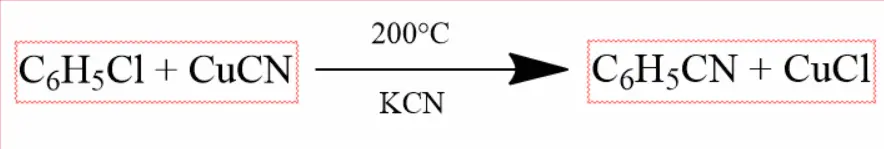
Wurtz Fittig Reaction –
Tolvine is formed when chlorobenzene is treated with methyl chloride in anhydrous ether in the presence of sodium.

Sulfonation –
On heating chlorobenzene with concentrated sulfuric acid, a mixture of o and p-chlorobenzene sulfonic acids is obtained.
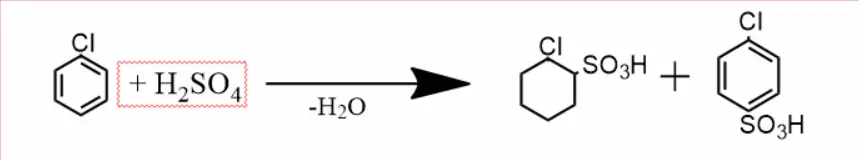
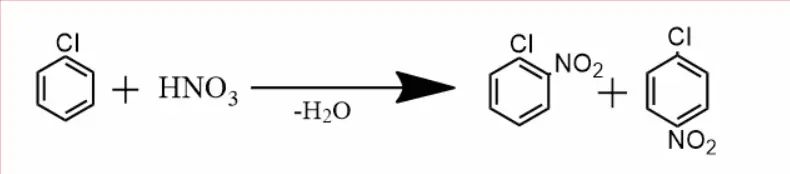
Nitrification –
Chlorobenzene reacts with nitric acid to give o-chlorobenzene nitro and p-Chloronitro benzene.
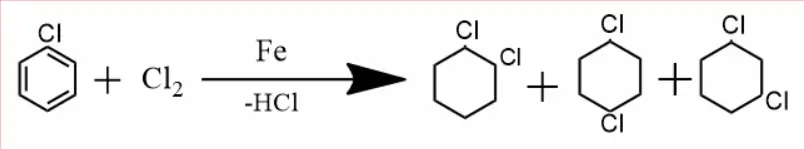
Halogenation –
When chlorobenzene is treated with halogens (halogen family elements) in the presence of iron, they form ortho, para, and meta.
Reaction with chloral –
On heating chlorobenzene with chloral in the presence of concentrated sulfuric acid (H2SO4), p, p-dichloro Diphenyl trichloroethane (DDT) is formed. DDT is a pesticide chemical.
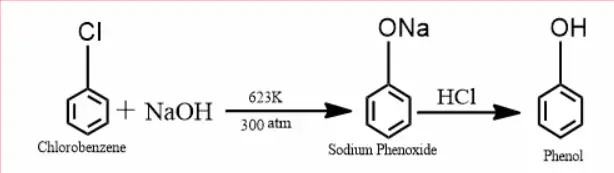
How to get phenol from chlorobenzene?
When chlorobenzene is treated with sodium hydroxide at 623 K and 320 atm pressure, sodium phenoxide is formed. Then, the sodium phenoxide is treated with hydrogen chloride to give phenol, and sodium chloride is released.
What Is The IUPAC Name Of Chlorobenzene?
Friends, the IUPAC name of Chlorobenzene is Chlorobenzene, it has different names like – Phenyl chloride and mono chlorobenzene.
What Is the Molar Mass Of Chlorobenzene?
The molar mass of chlorobenzene is 112.56 g/mol.
What Is The Density Of Chlorobenzene?
The density of Chlorobenzene is 1.11 g/cm3, liquid.
What Is the Use of Chlorobenzene?
Chlorobenzene makes DDT, Phenol, aromatic compounds, and medicines. Read chemical properties to know its more uses.
Lewis Dot Structure Of Chlorobenzene –
It is the Lewis dot structure of chlorobenzene.
Friends, in today’s article, we read about what is Chlorobenzene, how is chlorobenzene made, and what is the use of chlorobenzene, we have studied the answers to many such questions. So friends, if you like this article, share it on your social platform.
Thank you so much