Hello friends, today we will talk about Afbau’s Principle, friends, what is Aufbau’s principle? Before knowing this, we need to know about the Shell and sub-shell. So first, let us know about the Shell and sub-shell. Now know this, what do we have to do with the Aufbau principle? It will be easy to understand.
What are Shell and Subshell?
Definition of Shell and Subshell –
The electrons in an atom are arranged in different main energy levels called shells. The main energy levels are divided into sub-energy levels. It’s called a subshell.
Shells are divided into four subshells called s subshells, p subshells, d subshells, and f subshells, respectively. These subshells are divided into orbitals called s orbitals, p orbitals, d orbitals, and f orbitals, respectively.
There is 1 orbital in the s subshell. There are 3 orbitals in the p subshell. The d subshell has 5 orbitals, and the f subshell has 7 orbitals.
Any orbital can hold a maximum number of electrons of opposite spin.
The s subshell has a maximum of 2 electrons. Whereas the p subshell can have a maximum of 6 electrons, the d subshell can have a maximum of 10 electrons and the f subshell can have a maximum of 14 electrons. The electrons are filled in the atomic orbital.
Also, read: What is Atomic structure, Rutherford atomic model?
What is Aufbau’s Principle?
Aufbau Principle –
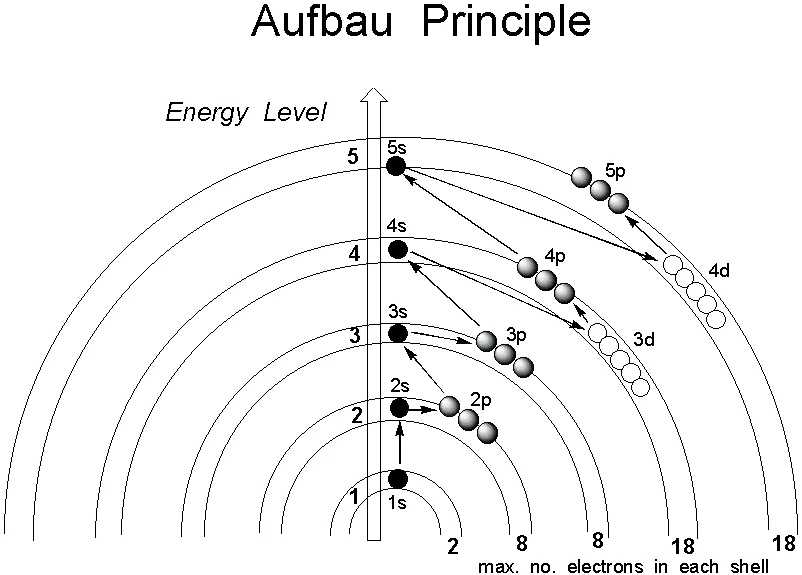
Aufbau is a German word that means to build, according to this law the electronic configuration of elements is made. aufbau principle is the law of making electronic configurations of elements.
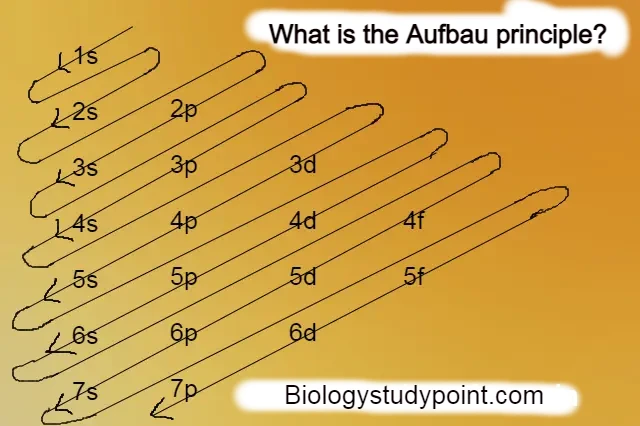
According to this principle, the arrangement of electrons in increasing order of energy level is called the Aufbau principle.
According to the Aufbau principle, the first electron enters the 1s orbital. After the 1s orbital is complete, electrons enter the 2s orbital. Similarly, as shown in the figure, electrons enter the 2p orbital. Similarly, electrons keep filling up gradually.
What is an electron cloud?
Electron Cloud –
H – electron cloud of the hydrogen atom –
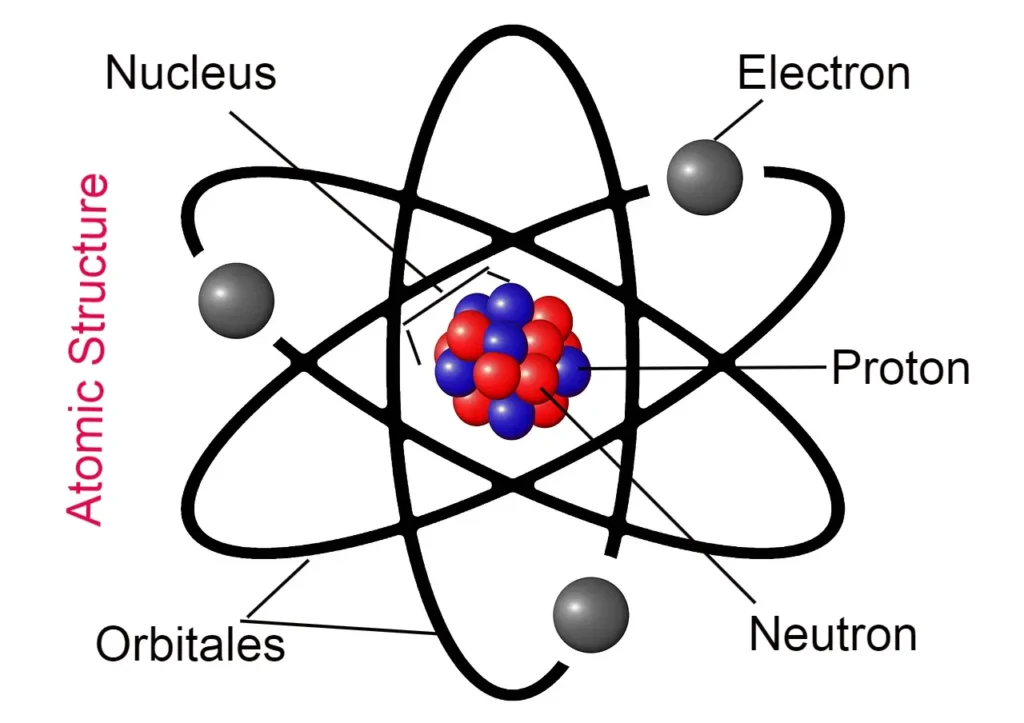
The electrons revolve around the nucleus of any atom at a rapid rate. As a result, a dense cloud of electrically negative charge of electrons is formed. Which is called the cloud of electrons. This dense electron is known as the atomic orbital.
What is an atomic orbital?
The area around the atomic nucleus, where electrons are most likely to be found. It is called an atomic orbital.
What is an electronic configuration?
Electronic Configuration –
The electronic configuration is done according to the inert code rule.
| Helium | Neon | Argon | Krypton | Xenon | Radon |
| 2He | 10Ne | 18Ar | 36Kr | 54Xe | 86Rn |
According to the Aufbau principle, the distribution of electrons is done in the subshell.
Shell
↑
4s
↙ ↘
Orbit Orbital
The electronic configuration according to inert gas.
7 = [He], 2s2, 2p3
5 = [He], 2s2, 2p1
13 = [Ne], 3s2, 3p1
17 = [Ne], 3s2, 3p5
21 = [Ar], 4s2, 3d1
In any atom, the distribution of electrons in the half-filled configuration and the fully-filled configuration is uniform. The value of internal repulsion force between electrons in a half-filled configuration and a full-filled configuration is minimal, and it is a stable configuration.
Half-filled configuration and full-filled configuration is a permanent arrangement. The d-subshell takes away electrons from the s-subshell to make its permanent configuration. Since the energy difference between the s subshell and the d subshell is very small. When the filling of electrons is started. So the value of internal electronic repulsion force increases.
To reduce this, the s-electron goes by jumping into the d-electron. This is the reason for the exception. This transfer of electrons takes place to form a symmetric configuration.
When an atom creates a cation. So the electron leaves the last shell and not the last subshell.
When an atom creates an anion. So the number of electrons increases, this electron is filled in the subshell and not in the shell.
Example-
26Fe = [Ar], 4s2, 3d6
26Fe+2 = [Ar], 4s0, 3d6
26Fe+3 = [Ar], 4s0, 3d5
29Cu = [Ar], 4s2, 3d9 [Wrong]
29Cu = [Ar], 4s1, 3d10 [Correct]
17Cl = [Ne], 3s2, 3p5
17Cl- = [Ne], 3s2, 3p6
How are electrons distributed in shells?
Distribution of Electrons in shells –
The distribution of electrons in shells is done according to the Bohr-Bury law. The maximum number of electrons in a shell is 2n2.
11Na = 1s2, 2s2, 2p2, 3s1
11Na = 2, 8, 1
17Cl = 1s2, 2s2, 2p6, 3s2, 3p5
17Cl = 2, 8, 7
32Ge = 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p2
32Ge = 2, 8, 18, 4
How are electrons distributed in subshells?
Distribution of electrons in subshell –
The distribution of electrons in subshells is done according to Afbau’s law. The energy value of the subshell depends on the (n+l) value. The order of the subshells is f, d, p, s but the principal quantum numbers are arranged in increasing order.
The value of (n+l)
| 1s, | 2s, | 2p 3s, | 3p 4s, | 3d 4p 5s, | 4d 5p 6s, | 4f 5d 6p 7s, | 5f 6d 7p 8s |
| 1 | 2 | 3 3 | 4 4 | 5 5 5 | 6 6 6 | 7 7 7 7 | 8 8 8 8 |
If (n+l) = 5 then what will be the number of orbitals?
m = total number of orbitals
m = 5+3+1
m = 9 orbitals
Position in Periodic Table –
The position of any element in the periodic table can be determined based on the following rules.
Rule 1: When the electronic configuration of an element is made, the value of the maximum principal quantum number tells the period of that element.
Rule 2: When the electronic configuration of an element is made, the differential electron or valence electron, or the last electron of the element enters the subshell. That is the block of that element.
Rule 3: When an element belongs to the s block and p block then that element will belong to subgroup A and if an element belongs to the d and f block then it will always belong to subgroup B.
Rule 4: When the element is related to the s block and p block, then in such case the number of electrons in the last orbit. That will be the group number of that element.
Rule 5: If an element belongs to a d-block, then in such case the sum of the last two orbital electrons is equal to -8, if this difference is 3, 4, 5, 6, 7 then the group number is the same.
If the difference is 8, 9, or 10 then the group number is 8, similarly, if this difference is 11, or 12 then the group number will be 1 and 2.
Rule 6: If element f belongs to block, then in such case the group number will always be third- B.
Example –
11Na = [Ne], 3s1
Period = Third
block = s
Group Number = First A
26Fe = [Ar] , 4s2, 3d6
Period = Four
block = d
Group number = 8
35 = [Ar] , 4s2, 3d10, 4p5
Period = Four
block = p
Group number = 8
80 = [Xe] , 6s2, 4f14, 5d10
Period = Six
block = d
Group number = 2+10+6+2-8 = 12
Group number = 2 – B
65 = [Xe], 6s2, 4f9
period = six
block = f
Group Number = Third – B
Exception to Aufbau principle –
24 = [Ar], 4s2, 3d4
= [Ar], 4s1, 3d5
period = four
block = d
Group number = 1+5+6+2-8 = 6
Group Number = Six – B
Conclusion –
Now let us see what things we have read in it.
We have come to know step by step about all these things about shells and subshells, the Aufbau principle, electron cloud, and electronic configuration. Important notes, distribution of electrons in shells, distribution of electrons in subshells, place, and rules in the periodic table.
Conclusion
Friends, I hope that you have liked the Aufbau principle and the given information related to it. Friends, if you liked this information, then please share it with your friends as much as possible. So that they can also benefit from it, I am sure that you will share it.
Thank you