Hello friends, today we will learn well about what is matter in this article, so let’s start without delay.
What is matter?
Matter –
- The matter is the thing that has mass and volume, called matter which we see, and feel.
- The matter has four basic elements.
- There is fire, air, water, and earth.
- For example, on burning moist green wood, smoke (air) + water vapor (water) + flame (fire) + residue gets converted into ash (earth).
Also, read – What is an Atom? Discovered, the atomic model
Now we will learn about the state of matter.
State of matter –
There are mainly five states of matter.
- Solid State
- Liquid State
- Gaseous state
- Plasma state
- Bose-Einstein Condensate state
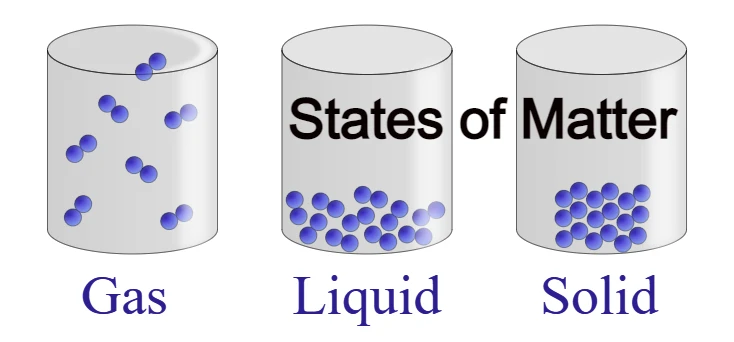
Solid State –
Those substances whose shape and volume are both fixed, then this state of matter is called solid state.
Examples – wood, iron, ice, silver, gold, glass, etc.

Liquid State –
Those physical states whose volume is fixed, but the shape is not fixed. So such a physical state is called a liquid state.
Example – water, oil, etc.
Gaseous state –
Those physical states whose volume and shape are both uncertain. But the vessel in which they are kept. If it assumes the same shape, then such a physical state is called a gaseous state.
Examples – air, smoke, vapor, etc.
Plasma state –
The plasma state is possible at very high temperatures, this state of matter is free, highly energetic, and a collection of charges, it contains negatively charged (e–) electrons and positively charged ions.
The plasma state is found due to the very high temperature of the Sun and the stars. For example, fluorescent tubes and neon bulbs.
Bose-Einstein Condensate state –
In 2001, American scientists Eric A. Cornell, Wolfgang Kettle, and Carl E. Weizmann received the Nobel Prize in Physics for cooling a gas with a density as low as one-millionth of the density of normal air at a very low temperature.
There are some such substances, which are found in all three states, solid, liquid, and gas. For example, water is found in the form of ice in the solid state, in the form of water in the liquid state, and the form of steam in the gaseous state.
What is the difference between solid, liquid, and gas states?
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Its size is fixed. | Its size is uncertain. The vessel in which it is kept. Takes the same shape. | Neither its shape is fixed, nor the volume of the vessel in which the gas is kept. Takes the same shape and volume. |
| Volume | Its volume is fixed. | Its volume is also fixed. | Its volume is not fixed. The vessel in which it is kept. Takes the same shape. |
| Blank space | There is very little space between molecules. | The space between its molecules is slightly larger than that of a solid. | It consists mostly of blank spaces. |
| Density | Its density is high. | Its density is less than that of a solid. | Its density is very low. |
| Flowability | It does not flow. | It flows. | Furthermore, it also flows. |
| Heat effect | It expands on heating. | It boils on heating (at high temperature). | Furthermore, it expands very fast on heating. |
| Floor | It doesn’t have to be flat. | The surface of a stationary liquid is always flat. | It does not have any bottom. |
| Pressure effect | It can be pressed very little. | It can be pressed more than concrete. | The gas may be overdressed. |
| Melting point and boiling point | It has a fixed melting point. | It has a fixed boiling point. | Furthermore, it has a definite critical temperature. |
| Example | Ice, wax, sulfur, iron, and other metals (except mercury), wood, paper, stone, etc. | Water, milk, oil, honey, mercury, etc. | Oxygen, air, nitrogen, hydrogen, and other gases. |
What is a mixture?
When two or more pure substances are mixed in any ratio, the substance formed is called a mixture.
The composition and properties of each part of the mixture are different.
For example, iron filings, sand, air, etc.
What is a compound?
When two or more elements are mixed in a certain proportion according to the mass, the substance formed is called a compound.
Each part of a compound has a definite composition but has different properties.
Such as – water (H2O), carbon dioxide (CO2), ammonia (NH3), calcium chloride (CaCl2), etc.
What is the difference between a mixture and a compound?
| Mixture | Compound |
|---|---|
| A mixture is formed by mixing two or more pure substances in any ratio. | Compounds are formed by mixing two or more elements in a fixed ratio by mass. |
| In this, the properties of the constituent substances remain present. | Its properties are different from the properties of the elements. |
| Its components can be separated by physical methods | Its components cannot be separated by physical methods. |
| There is no chemical change in its formation | A chemical change takes place in its formation. |
| Neither energy is needed nor produced to make it. | Energy is either needed to make it or it is generated. |
| It is often heterogeneous. (except a few) | It is a Homogeneous. |
| Its physical constants (melting point, boiling point, etc.) are not fixed. | Its physical constant is fixed. |
I hope you guys what is the matter, You must have liked the information given about it. Friends, if you liked this information, then please share it as much as possible. So that your friend can also benefit from it.
Thank you
Also, read –
What is Atomic structure? Rutherford atomic model.
What is Chemistry? Learn about it.